RegulatoryID
Navigate life sciences transparency requirements with ease
The most comprehensive, easy-to-use repository of global regulatory information, MedPro Systems RegulatoryID offers a simple, centralized source to efficiently track, filter and manage reporting requirements.
Researched, written and verified by our pharmaceutical and medical device compliance experts, RegulatoryID equips you to navigate Life Sciences transparency requirements with ease while reducing your risk of non-compliance and staying current with evolving regulations.
Your Guide to Global and U.S. Reporting Requirements
RegulatoryID delivers structured, searchable access to the full scope of transparency and compliance obligations. Explore the core areas we cover—so your team can track, manage and respond to regulatory changes with clarity and confidence.
US Aggregate Spend Reporting
- Transparency Reporting
- Gift Bans and Limitation
- Field Rep Registration and Licensure
- Compliance Program Requirements
Global Aggregate Spend Reporting
- Centralized Disclosure Compendium of 50+ Countries
- Industry Association-Based Requirements (including EFPIA & MedTech Europe)
- National Laws, Industry Codes, Guidance Documents and Templates
- Gift Bans & Limitations
Save Time by Searching and Sorting the Information you Need in Seconds
Eliminate the need to manually track down laws and regulatory content from industry, legal and government sources.
Quickly search, filter and highlight the information most relevant to your business using filters such as:
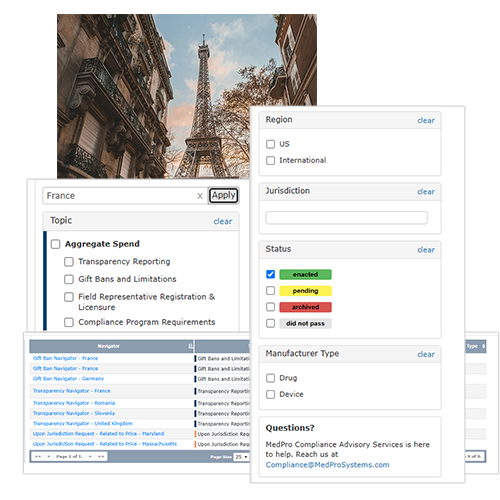
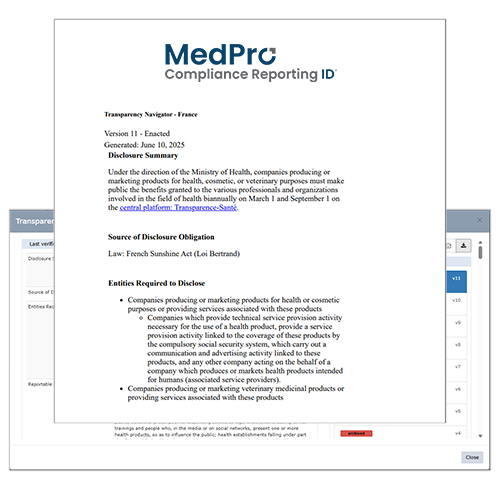
Curate Results to Match your Preferred Level of Detail
All resources are structured and formatted intuitively with:
- Visual Summaries Quickly reference high-level requirements across all jurisdictions
in scope per topic. - Detailed Compilations (Navigators) outline requirements from disparate laws, regulations and guidance documents in one convenient location, with links to authoritative sources for each topic and jurisdiction. As Navigators are updated, versions can be compared side by side with highlighted changes for quick review.
Stay Up to Date with Proactive Notifications
Receive timely regulatory alerts and email reminders about evolving requirements, deadlines and updates sent directly to your inbox so you never miss a change.

Explore More
Leverage our end-to-end global platform to meet all your disclosure needs with configurable transparency product categories and support from our dedicated, nimble, forward-looking team of industry veterans.

Seamless US federal, state, local and international reporting platform.

Seamless transparency data capture into T&E Platforms.

Ready to Streamline Compliance?
Book a demo with our regulatory experts and see for yourself how easy it is to navigate life sciences transparency requirements with our comprehensive regulatory solution.
